Real-Time PCR Kits
HLA-B
The human leukocyte antigen (HLA) system is a gene complex that encodes the major histocompatibility complex (MHC) proteins in humans. These cell-surface proteins are essential for regulating the immune system.
HLA-B27 is critical for diagnosing ankylosing spondylitis (AS) and related spondyloarthropathies, playing a significant role in the pathogenesis of these diseases. Ankylosing spondylitis has several subtypes, and its prevalence varies among different ethnic groups.
HLA-B57:01 is a genetic variation associated with hypersensitivity to the antiretroviral (ARV) drug abacavir, commonly used in combination antiretroviral therapy (cART) for treating HIV. Screening patients for the presence of HLA-B57:01 before initiating abacavir therapy significantly reduces the risk of hypersensitivity reactions (HSRs).
Features
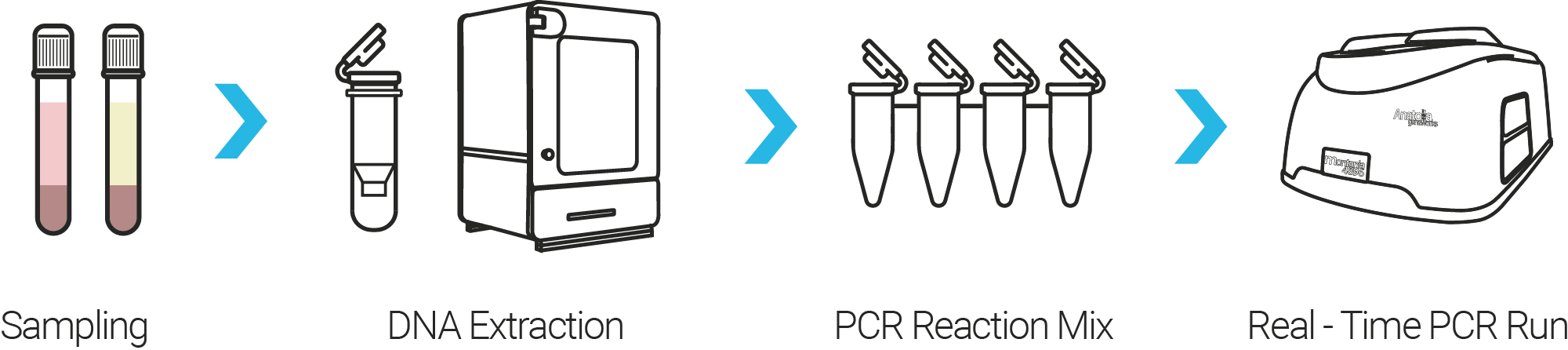
Workflow
Technical Specifications
The channels and sample types indicated in this table may vary depending on the kits (singleplex/multiplex). Detailed information on the associated kits can be found below.
| Thermal Protocol | Single thermal protocol for all parameters |
| Sample Types | Whole blood and blood culture |
| Shelf Life | 18 Months |
| Channels | FAM, HEX, Cy5 |
| Shipping / Storage | (-90°C)-(-20°C) / -20°C |
HLA-B Kits
Bosphore HLAB27 Detection Kit v1, is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore HLAB27 Detection Kit v1 detects HLAB27 allele existence in human biological samples. HLAB27 allele is amplified and fluorescence detection is accomplished using the FAM filter. For the situations that HLAB27 allele is absent in the sample, a housekeeping gene GAPDH is also amplified as internal control. Fluorescence detection of GAPDH is accomplished using the Cy5 filter in order to prove that PCR reaction is performed accurately.
Bosphore HLAB27 Detection Kit v2 detects HLAB27 allele existence in human biological samples. HLAB27 allele is amplified and fluorescence detection is accomplished using the FAM filter. For the situations that HLAB27 allele is absent in the sample, a housekeeping gene GAPDH is also amplified as internal control. Fluorescence detection of GAPDH is accomplished using the Cy5 filter in order to prove that PCR reaction is performed accurately.
Only a single incubation with the EX-Tract DNA solution is enough to prepare the samples for qPCR. There is no need to apply an external DNA isolation.
Bosphore HLA-B57:01 Detection Kit v1, is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore HLA-B57:01 Detection Kit v1 detects HLA-B*57:01 allele existence in exon 2 and exon 3 in human biological samples.
In the 1st well, HLA-B*57:01 allele in exon 2 is amplified and fluorescence detection is accomplished using the FAM filter.
In the 2nd well, HLA-B*57:01 allele in exon3 is amplified and fluorescence detection is accomplished using the HEX filter.
For the situations that HLA-B*57:01 allele is absent in the sample, a housekeeping gene GAPDH is also amplified as internal control in both wells. Fluorescence detection of GAPDH is accomplished using the Cy5 filter in order to prove that PCR reaction is performed accurately.




