The rising prevalence of infectious diseases has increased demand for simple, rapid in-vitro diagnostics (IVDs), resulting in a surge in public adoption of the lateral flow assay format in recent years. Lateral flow assays have several advantages compared to other technologies in the IVD field that they are inexpensive to produce, scalable, instrumentation-free to interpret, user-friendly without formal training, and capable of providing reliable results at home or in a laboratory setting. Rapid lateral flow test systems are easy-to-use, disposable diagnostic devices that can yield results in a short time (<30 minutes) by analyzing biomarkers in samples such as nasal swabs, saliva, blood, urine, and food.
Rapid Antigen Tests
Features
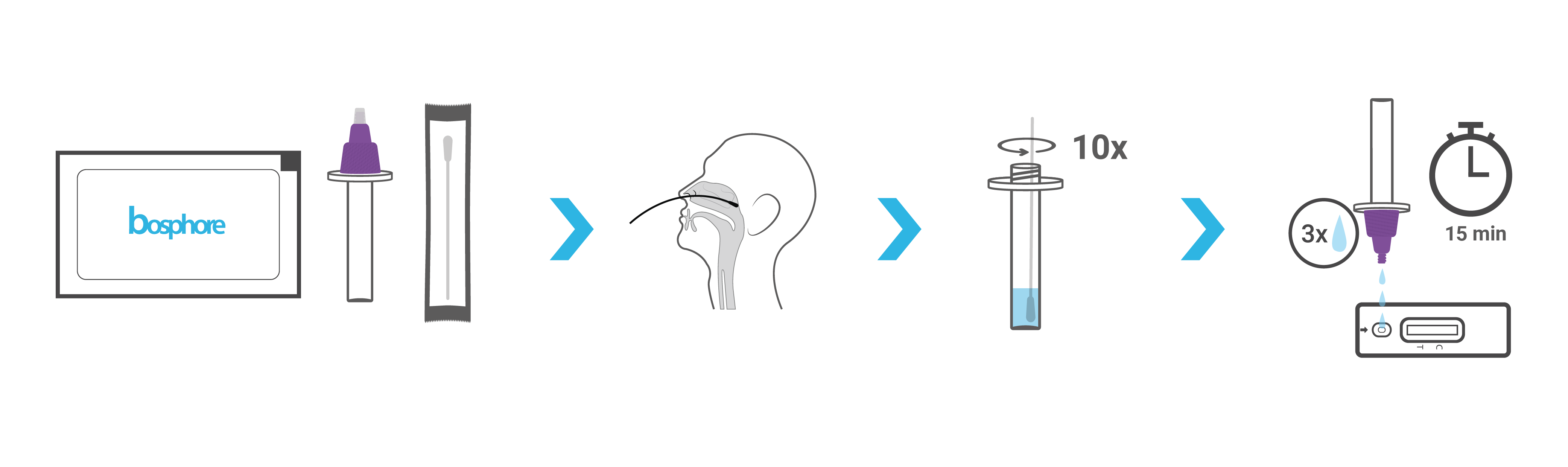
Workflow
Technical Specifications
The sample types indicated in this table may vary depending on the kits (singleplex/multiplex). Detailed information on the associated kits can be found below.
| Sample Types | Nasopharyngeal and Oropharyngeal Swab (For Respiratory Pathogens) Fecal Sample (For Gastrointestinal Diseases) |
| Shelf Life | 2 years |
| Shipping / Storage | 2-30°C |
| Operation Time | 15 min |
Rapid Antigen Test Kits
2019 Novel Coronavirus (2019-nCoV), which belongs to the same family as the pathogen that causes severe acute respiratory syndrome (SARS), is first identified as the cause of a respiratory illness outbreak detected in Wuhan, China.
The main symptoms can include fever, cough, and difficulty breathing ranging from mild symptoms to severe illness and death. Cases of severe infection can result in pneumonia, kidney failure, and death.
Bosphore SARS-CoV-2 Rapid Antigen Test Kit is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal swabs from individuals suspected of COVID-19 by their healthcare provider within the first seven days of symptom onset.
This test is used only by professionals for the diagnosis of SARS-CoV-2 infection in clinical laboratories or for point-of-care testing. The test is not for personal use.
Kit Components
- Test Cassette
- Extraction Buffer
- Instructions for Use
- Disposable swab
Bosphore Flu A/B Rapid Antigen Test Kit v1 is a lateral flow immunoassay intended for the qualitative detection of the nucleocapsid protein antigens from Influenza A and Influenza B in human nasopharyngeal swab specimens. The test is designed for use by healthcare professionals in clinical laboratories and point-of-care (POC) settings to aid in the diagnosis of Influenza A and Influenza B infections in individuals who are suspected of infection within the first seven days of symptom onset.
Bosphore RSV Rapid Antigen Test Kit v1 is a lateral flow immunoassay intended for the qualitative detection of RSV F (Fusion protein) and G (Glycoprotein) protein antigens in human nasopharyngeal and anterior nasal swab specimens. The test is designed for use by healthcare professionals in clinical laboratories and point-of-care (POC) settings to aid in the diagnosis of RSV infection in individuals who are suspected of infection within the first few days of symptom onset.
Bosphore Strep A Rapid Antigen Test Kit v1 is a lateral flow immunoassay intended for the qualitative detection of Streptococcus pyogenes (Group A β-hemolytic Streptococcus, Strep A) group A streptococcal antigen in human oropharyngeal swab specimens. The test is designed for use by healthcare professionals in clinical laboratories and point-of-care (POC) settings to aid in the diagnosis of Strep A infection in individuals who are suspected of infection within the first seven days of symptom onset.
Bosphore Adenovirus Rapid Antigen Test Kit v1 is a lateral flow immunoassay intended for the qualitative detection of Adenovirus Hexon protein antigen in human fecal specimens. The test is designed for use by healthcare professionals in clinical laboratories and point-of-care (POC) settings to aid in the diagnosis of Adenovirus infection in individuals who are suspected of infection within the first seven days of symptom onset.
Bosphore Rotavirus Rapid Antigen Test Kit v1 is a lateral flow immunoassay intended for the qualitative detection of Rotavirus VP6 antigen in human fecal specimens. The test is designed for use by healthcare professionals in clinical laboratories and point-of-care (POC) settings to aid in the diagnosis of Rotavirus infection in individuals who are suspected of infection within the first seven days of symptom onset.
Bosphore RotaAdeno Virus Rapid Antigen Test Kit v1 is a lateral flow immunoassay intended for the qualitative detection of the hexon protein antigen of Adenoviruses and the VP6 antigen of Rotaviruses in human fecal specimens. The test is designed for use by healthcare professionals in clinical laboratories and point-of-care (POC) settings to aid in the diagnosis of Rotavirus and Adenovirus infections in individuals who are suspected of infection within the first seven days of symptom onset.
Bosphore H. pylori Rapid Antigen Test Kit v1 is a lateral flow immunoassay intended for the qualitative detection of Helicobacter pylori antigens in human fecal specimens. The test is designed for use by healthcare professionals in clinical laboratories and point-of-care (POC) settings to aid in the diagnosis of Helicobacter pylori infection in individuals who are suspected of infection within the first seven days of symptom onset.
Bosphore Fecal Occult Blood (FOB) Rapid Antigen Test Kit v1 is a lateral flow immunoassay intended for the qualitative detection of human hemoglobin (occult blood) in fecal samples, indicating potential gastrointestinal bleeding. The test is designed for use by healthcare professionals in clinical laboratories and point-of-care (POC) settings, specifically for patients in the early stages of symptoms where gastrointestinal bleeding is suspected.
