Real-Time PCR Kits
Thrombophilia
Genetic diseases occur as a result of anomalies in people’s DNA. These anomalies can occur on a very wide scale, from a small mutation in a gene to the addition or removal of a new chromosome to the chromosomes.
Genetic tests are tests that directly look at the presence or absence of the relevant gene and/or the major important protein for the disease, again at the gene level or biochemically, and inform and guide clinicians to diagnose disorders. The test material required for these tests can be obtained from the person’s blood, body fluids or tissue pieces such as biopsy material.
Real-Time PCR is more used and preferred than other genetic tests because it is advantageous in terms of precision, speed and efficiency of diagnosis.
Features
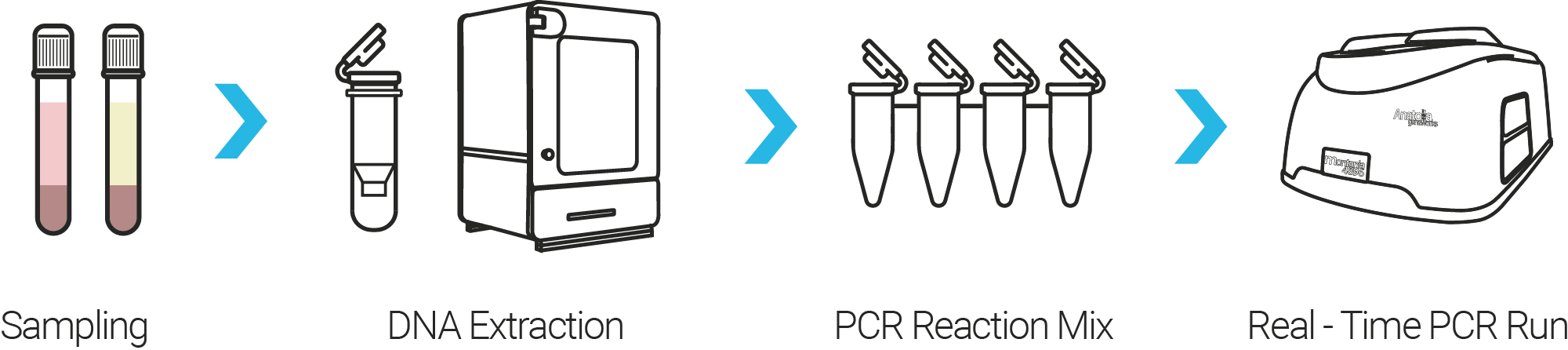
Workflow
Technical Specifications
The channels and sample types indicated in this table may vary depending on the kits (singleplex/multiplex). Detailed information on the associated kits can be found below.
| Thermal Protocol | Single thermal protocol for all parameters |
| Sample Types | Whole blood |
| Shelf Life | 18 Months |
| Channels | FAM, HEX, Texas Red, Cy5 |
| Shipping / Storage | (-90°C)-(-20°C) / -20°C |
Thrombophilia Kits
Bosphore FII-FVL Detection Kit v1 detects Factor II mutation in the 3’UTR region of FII gene; namely G20210A (prothrombin mutation / a change of guanine to adenine) and Factor V Leiden mutations; namely G1691A (a change of glutamic acid to arginine) in human biological samples.
Wild type FII gene is amplified and fluorescence detection is accomplished using Cy5 filter. FII mutation is amplified and fluorescence detection is accomplished using Texas Red filter. Wild type F5 allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant F5 allele is amplified and fluorescence detection is accomplished using the FAM filter.
Bosphore FII-FVL Detection Kit v2 (Fast Extraction) detects Factor II mutation in the 3’UTR region of FII gene; namely G20210A (prothrombin mutation / a change of guanine to adenine) and Factor V Leiden mutations; namely G1691A (a change of glutamic acid to arginine) in human biological samples.
Wild type FII gene is amplified and fluorescence detection is accomplished using Cy5 filter. FII mutation is amplified and fluorescence detection is accomplished using Texas RED filter. Wild type F5 allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant F5 allele is amplified and fluorescence detection is accomplished using the FAM filter.
Shipping possible also on dry ice.
Bosphore FV H1299R Detection Kit v1, is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore FV H1299R Detection Kit v1 detects the A4070G mutation (H1299R a change of histidine to arginine at position 1299) of the F5 gene in human biological samples. Wild type F5 allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant F5 allele is amplified and fluorescence detection is accomplished using the FAM filter.
Bosphore FV H1299R Detection Kit v4 detects the A4070G mutation (H1299R a change of histidine to arginine at position 1299) of the F5 gene in human biological samples. Wild type F5 allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant F5 allele is amplified and fluorescence detection is accomplished using the FAM filter.
The whole blood samples are incubated with Ex-Tract DNA solution found in the kit content in order to be prepared for PCR set-up. There is no need for additional DNA extraction step.
Shipping possible also on dry ice
Bosphore FVC Detection Kit v1, is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore FVC Detection Kit v1 detects Factor V Cambridge mutation in human F5 gene (a change of arginine to threonine in 306th amino acid) in human biological samples. Wild type F5 allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant F5 allele is amplified and fluorescence detection is accomplished using the FAM filter.
Bosphore FVC Detection Kit v2 (Fast Extraction), is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore FVC Detection Kit v2 (Fast Extraction) detects Factor V Cambridge mutation in human F5 gene (a change of arginine to threonine in 306th amino acid) in human biological samples. Wild type F5 allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant F5 allele is amplified and fluorescence detection is accomplished using the FAM filter.
Shipping possible also on dry ice
Bosphore FVL Detection Kit v3 detects Factor V Leiden mutation; namely G1691A (a change of glutamic acid to arginine) in human biological samples. Wild type F5 allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant F5 allele is amplified and fluorescence detection is accomplished using the FAM filter.
Bosphore FVL Detection Kit v4 detects Factor V Leiden mutation; namely G1691A (a change of glutamic acid to arginine) in human biological samples. Wild type F5 allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant F5 allele is amplified and fluorescence detection is accomplished using the FAM filter.
Shipping possible also on dry ice
Bosphore FXIII Detection Kit v3 detects Factor XIII mutation; a point mutation (Guanine base replacement by Thymine) found in the 2nd exon of FXIII which leads to conversion of Valine amino acid to Leucine within codon 34 (FXIIIVal34Leu) in human biological samples. Wild type FXIII allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant FXIII allele is amplified and fluorescence detection is accomplished using the FAM filter.
Bosphore FXIII Detection Kit v4 detects Factor XIII mutation; a point mutation (Guanine base replacement by Thymine) found in the 2nd exon of FXIII which leads to conversion of Valine amino acid to Leucine within codon 34 (FXIIIVal34Leu) in human biological samples. Wild type FXIII allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant FXIII allele is amplified and fluorescence detection is accomplished using the FAM filter.
Shipping possible also on dry ice
Bosphore MTHFR A1298C Detection Kit v3, is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore MTHFR A1298C Detection Kit v3 detects MTHFR mutation; namely A1298C (a change of adenine nucleotide with the cytosine at position 1298) in human biological samples. Wild type MTHFR allele is amplified and fluorescence detection is accomplished using the HEX filter. Allele with MTHFR polymorphism is amplified and fluorescence detection is accomplished using the FAM filter.
Bosphore MTHFR A1298C Detection Kit v4 detects MTHFR mutation; namely A1298C (a change of adenine nucleotide with the cytosine at position 1298) in human biological samples. Wild type MTHFR allele is amplified and fluorescence detection is accomplished using the HEX filter. Allele with MTHFR polymorphism is amplified and fluorescence detection is accomplished using the FAM filter.
The whole blood samples are incubated with Ex-Tract DNA solution found in the kit content in order to be prepared for PCR set-up. There is no need for additional DNA extraction step.
Shipping possible also on dry ice
Bosphore MTHFR C677T Detection Kit v3 detects MTHFR mutation; namely C677T (a change of cytosine nucleotide with the thymine at position 677) in human biological samples. Wild type MTHFR allele is amplified and fluorescence detection is accomplished using the HEX filter. Allele with MTHFR polymorphism is amplified and fluorescence detection is accomplished using the FAM filter.
Bosphore MTHFR C677T Detection Kit v4 detects MTHFR mutation; namely C677T (a change of cytosine nucleotide with the thymine at position 677) in human biological samples. Wild type MTHFR allele is amplified and fluorescence detection is accomplished using the HEX filter. Allele with MTHFR polymorphism is amplified and fluorescence detection is accomplished using the FAM filter.
The whole blood samples are incubated with Ex-Tract DNA solution found in the kit content in order to be prepared for PCR set-up. There is no need for additional DNA extraction step.
Shipping possible also on dry ice
Bosphore MTHFR 677-1298 Detection Kit v1 is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore MTHFR 677-1298 Detection Kit v1 detects MTHFR mutation; namely A1298C (a change of adenine nucleotide with the cytosine at position 1298) and MTHFR mutation; namely C677T (a change of cytosine nucleotide with the thymine at position 677) in human biological samples.
Wild type MTHFR C677T gene is amplified and fluorescence detection is accomplished using the HEX filter. Mutant MTHFR C677T gene is amplified and fluorescence detection is accomplished using the FAM filter.
Bosphore MTHFR 677-1298 Detection Kit v2 detects MTHFR C677T mutation (a change of cytosine nucleotide to thymine at position 677), and MTHFR A1298C mutation (a change of adenine nucleotide to cytosine at position 1298) in human biological samples. Wild type MTHFR A1298C gene is amplified and fluorescence detection is accomplished using Cy5 filter. Mutant MTHFR A1298C gene is amplified and fluorescence detection is accomplished using Texas Red filter. Wild type MTHFR C677T gene is amplified and fluorescence detection is accomplished using the HEX filter. Mutant MTHFR C677T gene is amplified and fluorescence detection is accomplished using the FAM filter.
Whole blood samples are incubated with Ex-Tract DNA solution found in the kit content in order to be prepared for PCR set-up. There is no need for additional DNA extraction step.
Shipping possible also on dry ice
Bosphore PAI-I 4G/5G Detection Kit v1 detects 4G/5G sequence length polymorphism which is formed by guanosine insertion/deletion variation at 675 bp upstream in PAI-I gene. PAI-I 4G allele is amplified and fluorescence detection is accomplished using the FAM filter. PAI-I 5G allele is amplified and fluorescence detection is accomplished using the CY5 filter.
Bosphore PAI-I 844 Detection Kit v1, is a Real-Time PCR based in-vitro diagnostic medical device, IVD CE marked according to 98/79/EC Directive. Bosphore PAI-I 844 Detection Kit v1 detects-844 guanine (G) adenine (A) replacement polymorphism in PAI-I gene. PAI-I G allele is amplified and fluorescence detection is accomplished using the FAM filter. PAI-I A allele is amplified and fluorescence detection is accomplished using the HEX filter.
Bosphore Prothrombin Detection Kit v3 detects Factor II mutation in the 3’UTR region of FII gene; namely G20210A (prothrombin mutation / a change of guanine to adenine) in human biological samples. Wild type FII gene is amplified and fluorescence detection is accomplished using the HEX filter. FII mutation is amplified and fluorescence detection is accomplished using the FAM filter.
Bosphore Prothrombin Detection Kit v4 detects Factor II mutation in the 3’UTR region of FII gene; namely G20210A (prothrombin mutation / a change of guanine to adenine) in human biological samples. Wild type FII gene is amplified and fluorescence detection is accomplished using the HEX filter. FII mutation is amplified and fluorescence detection is accomplished using the FAM filter.
Shipping possible also on dry ice
Bosphore Thrombophilia Panel Kit v1 detects Factor II mutation in the 3’UTR region of FII gene; namely G20210A (prothrombin mutation /a change of guanine to adenine) and Factor V Leiden mutations; namely G1691A (a change of glutamic acid to arginine), MTHFR C677T mutation (a change of cytosine nucleotide to thymine at position 677), and MTHFR A1298C mutation (a change of adenine nucleotide to cytosine at position 1298) in human biological samples.
In the first PCR tube with PCR Master Mix 1, Wild type FII gene is amplified and fluorescence detection is accomplished using Cy5 filter. FII mutation is amplified and fluorescence detection is accomplished using Texas RED filter. Wild type F5 allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant F5 allele is amplified and fluorescence detection is accomplished using the FAM filter.
In the second PCR tube with PCR Master Mix 2, Wild type MTHFR A1298C gene is amplified and fluorescence detection is accomplished using Cy5 filter. Mutant MTHFR A1298C gene is amplified and fluorescence detection is accomplished using Texas RED filter. Wild type MTHFR C677T gene is amplified and fluorescence detection is accomplished using the HEX filter. Mutant MTHFR C677T gene is amplified and fluorescence detection is accomplished using the FAM filter.
*Fast extraction option available
Bosphore Thrombophilia Panel Kit v2 (Fast Extraction) detects Factor II mutation in the 3’UTR region of FII gene; namely G20210A (prothrombin mutation /a change of guanine to adenine) and Factor V Leiden mutations; namely G1691A (a change of glutamic acid to arginine), MTHFR C677T mutation (a change of cytosine nucleotide to thymine at position 677), and MTHFR A1298C mutation (a change of adenine nucleotide to cytosine at position 1298) in human biological samples.
In the first PCR tube with PCR Master Mix 1, Wild type FII gene is amplified and fluorescence detection is accomplished using Cy5 filter. FII mutation is amplified and fluorescence detection is accomplished using Texas Red filter. Wild type F5 allele is amplified and fluorescence detection is accomplished using the HEX filter. Mutant F5 allele is amplified and fluorescence detection is accomplished using the FAM filter.
In the second PCR tube with PCR Master Mix 2, Wild type MTHFR A1298C gene is amplified and fluorescence detection is accomplished using Cy5 filter. Mutant MTHFR A1298C gene is amplified and fluorescence detection is accomplished using Texas RED filter. Wild type MTHFR C677T gene is amplified and fluorescence detection is accomplished using the HEX filter. Mutant MTHFR C677T gene is amplified and fluorescence detection is accomplished using the FAM filter.
The whole blood samples are incubated with EX-Tract DNA solution found in the kit content in order to be prepared for PCR set-up. There is no need for additional DNA extraction step.
Shipping possible also on dry ice
*Fast extraction option available
Bosphore Thrombophilia Panel Kit v3 detects Factor II mutation in the 3’UTR region of FII gene; namely G20210A (Prothrombin mutation/a change of guanine to adenine) and Factor V Leiden mutations; namely G1691A (a change of glutamic acid to arginine), MTHFR C677T mutation (a change of cytosine nucleotide to thymine at position 677), and MTHFR A1298C mutation (a change of adenine nucleotide to cytosine at position 1298) in human whole blood samples.
In the first PCR tube with PCR Master Mix 1, Wild type MTHFR C677T gene is amplified, and fluorescence detection is accomplished using the HEX filter. Mutant MTHFR C677T gene is amplified, and fluorescence detection is accomplished using the FAM filter. Wild type MTHFR A1298C gene is amplified, and fluorescence detection is accomplished using Cy5 filter. Mutant MTHFR A1298C gene is amplified, and fluorescence detection is accomplished using Texas RED filter.
In the second PCR tube with PCR Master Mix 2, Wild type F5 allele is amplified, and fluorescence detection is accomplished using the HEX filter. Mutant F5 allele is amplified, and fluorescence detection is accomplished using the FAM filter. Wild type FII gene is amplified, and fluorescence detection is accomplished using Cy5 filter. Mutant FII mutation is amplified, and fluorescence detection is accomplished using Texas RED filter.
*Fast extraction option available




