Anatolia has achieved a significant milestone by becoming the first molecular diagnostics manufacturer in Türkiye to obtain the IVDR certification. This certificate was granted following a successful audit conducted by the EU-authorized Notified Body QMD Services GmbH.
As Anatolia Geneworks, we are aware of our responsibility to healthcare. We see the IVDR transition period as a great opportunity to further increase our quality standards and support our end users better.
This achievement underscores Anatolia Geneworks’ commitment to maintaining the highest standards in molecular diagnostics. Our dedication to excellence has enabled us to receive IVD-CE Certification within the scope of IVDR for high-risk molecular diagnostic products.
What is IVDR? – A Quick Guide
In vitro diagnostic (IVD) medical devices play a crucial role in analysing human samples for diagnostic purposes. Given the direct impact of their results on patient well-being and treatment options, ensuring the safety and reliability of these devices is paramount.
IVDR, or the In Vitro Diagnostic Medical Devices Regulation (EU) 2017/746, replaced the previous directive (Directive 98/79/EC known as IVDD) on May 25, 2017. It sets forth regulations that IVD products must adhere to receive IVD CE marking and be permitted on the EU market.
Why IVDR is Important?
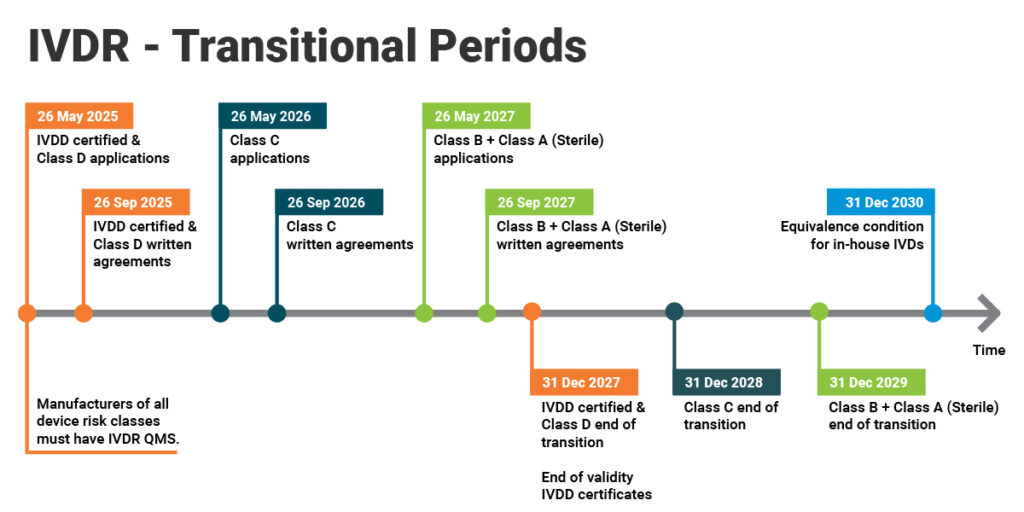
The transition period for IVDR implementation varies based on the classification of IVD devices and additional conditions outlined in IVDR 2017/746. Manufacturers must comply with IVDR requirements within the specified timelines to continue accessing the EU market.
Timeline of IVDR
The transition period depends on the class of IVD devices under the Directive and Regulation 2017/746 and additional conditions.